Selecting catalysts for marine fuels production
Accelerated activity and stability testing of hydrotreating catalysts (from PTQ Magazine - Digital Refining 2021 Q2)
ABRAR HAKEEM | Q8 Research
ED OUWERKERK | Catalyst Intelligence
Catalyst activity and stability are important factors in boosting the refining margin of units like reformers, hydrocrackers, and hydrotreating units. A refinery has little influence on feedstock and product prices, but can increase its margin by selecting the best catalyst for the unit configuration and by wisely planning its activity over the cycle.
A western European refiner wanted to select a catalyst best suited to its 100 m3 single bed hydrodesulphurisation (HDS) reactor operating at medium pressure with the aim of producing marine fuels. The aim is to produce fuels in three separate modes using three different Urals feed cuts. The main product components using three different feed cuts are: 50 ppm sulphur industrial gasoil (IGO), 800 ppm sulphur marine gasoil (MGO), and 4500 ppm sulphur low sulphur fuel oil (LSFO). In view of the size and duration of the catalyst sup- ply contract, the refinery decided to perform catalyst testing and not base its catalyst selection on vendor claims alone.
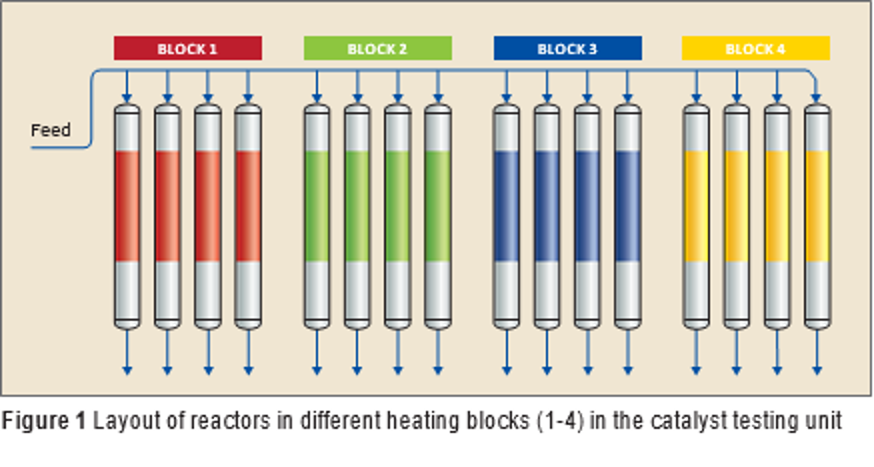
Q8 Research together with Catalyst Intelligence designed an experimental protocol for catalyst testing which guided the selection of the best performing catalyst. The catalyst testing was performed at Q8 Research under actual process conditions with actual feedstock used in the refinery.
A maximum cycle length for the catalyst is highly desirable. Moreover, it is beneficial for the refinery to have lower hydrogen consumption and at the same time meet all product specifications. The catalysts used for hydrotreating have a lifetime ranging from one to six years, depending on the composition of the feed (gasoil, vacuum gasoil, atmospheric resid, and so on)and process conditions (pressure, hydrogen/oil ratio, temperature, LHSV, hydrogen purity) used in the reactor.
Deactivation of hydrotreating catalysts occurs typically due to coke formation, metals deposition, and sintering of active sites.1 In the case of processing middle distillates (kerosene or gasoil), the metal con- tent of the feed is low and catalyst deactivation occurs most likely due to coke deposition over its lifetime.(2,3) In the case of heavier feedstocks (atmospheric residue, vacuum resid), catalyst deactivation occurs most likely by both metal deposition on catalysts and coke formation. Moreover, hydroprocessing catalyst deactivation is possible for all feeds due to the sintering of active sites over its lifetime. Sintering of active sites is directly related to temperature and can be enhanced when the reactors are operated at higher temperatures near to the end of life of the catalyst, or at high temperatures during operations, caused by upsets in the commercial reactor.To evaluate hydroprocessing catalysts’ stability, it is essential to perform an accelerated aging test, as it is not practically feasible to run a test for the exact lifetime of the catalyst.
However, the parameters used to design the accelerated test must be within the operating window of the actual process conditions used in the refinery.The term accelerated deactivation has no strict definition for a catalyst and can apply to different sets of process conditions used in a pilot unit, depending on the type of test. Many times, accelerated tests are designed with process variables which are never experienced in a real process and can result in different deactivation mechanisms that may not be relevant for actual stability comparison between the catalysts.(4) The term completely loses its purpose when it is referred to without indicating the actual process conditions (pressure, temperature, hydrogen/oil, LHSV) at which the respective catalysts were operated. (1,5) The experimental set-up in our case is designed in such a way that performance feedback at start of run (SOR) conditions is obtained from separate parallel reactors, and accelerated deactivation feedback is obtained from another set of parallel reactors. The conditions selected for accelerated deactivation are within the operating range of a commercial hydrotreatment reactor. Moreover, the design of the experiment allows a comparison of differently operated reactors under uniform hydrotreatment conditions at the end of the test.
Experimental
A 16-flow parallel reactor unit was used to perform catalyst testing. It has four different heating blocks (see Figure 1). Each heating block contains four reactors which can be set at different temperature to allow testing of multiple process conditions simultaneously. All of the experiments were performed at the same pressure, hydrogen purity, liquid hourly space velocity (LHSV), and hydrogen/oil ratio as is used in an actual reactor in the refinery.
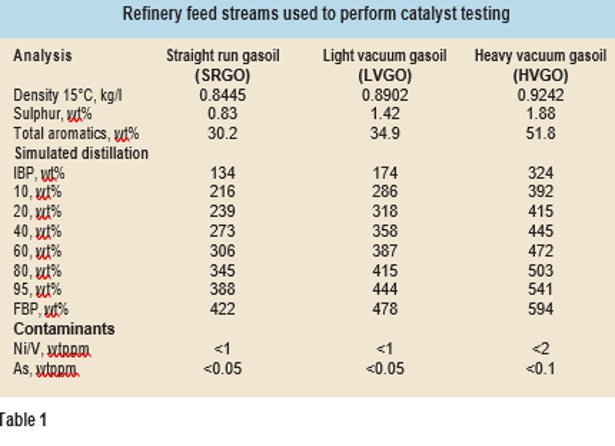
In order to have equal internal mass transfer influence on catalyst performance from different vendors, the catalyst extrudates as received were sorted in a length range of 2-4 mm. A customised robot is used at Q8 Research to perform catalyst sort- ing and size distribution analysis. This is an important step, as smaller catalyst particles allow more surface area and hence higher activity. Uniform size distribution is critical to provide precise comparison of catalyst activity, especially when using small scale reactors (<20 ml) for testing commercial catalysts from different vendors. Higher amounts of poisons (metals) and other foulants were absent from the feedstocks (see Table 1), so no hydrodemetallisation catalyst beds were loaded into the reactors.
Hydrotreatment catalysts from two different vendors (CatY and CatZ) were compared to evaluate their performance. The catalysts were loaded in 8 mm ID reactors and packed with inert silicon car- bide to ensure that plug flow criteria are maintained (no bypassing and limited axial dispersion). Three different feeds (see Table 1) were used to compare the performance of the catalysts under typical refinery process conditions. A common wetting and activation/sulphiding procedure was implemented and agreed with all the catalyst ven- dors. Dimethyl disulphide (DMDS) was added (3 wt%) to straight run gasoil for catalyst sulphidation/ activation. Catalyst activation was followed by a SOR temperature of 360°C using straight run gasoil (SRGO) feed.
Design of experiment
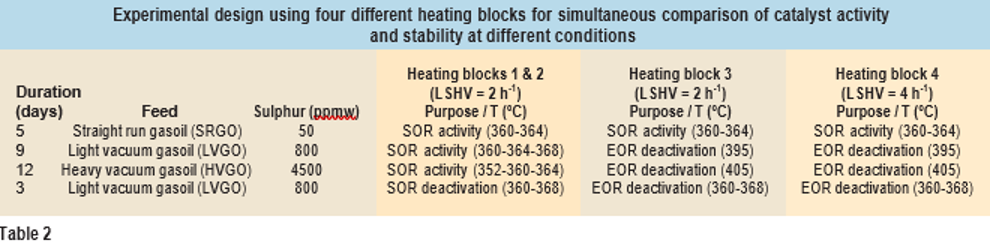
The experiment was designed in such a way that the activity as well as the stability of the catalysts could be compared within a relatively short period (30 days). For comparison of activity, heating blocks 1 and 2 were run close to the SOR temperature for different feeds. For insight into catalyst deactivation, conditions were repeated using LVGO feed (see Table 2). During commercial operation, catalyst deactivation is measured by an increase in the required weighted average bed temperature (WABT) to meet the required sulphur specification. Typical deactivation rates in middle distillate hydrotreatment is around a 0.5-2°C increase in WABT per month. To measure this deactivation with some accuracy, a normal test programme would typically require three months or longer. In parallel, heating blocks 3 and 4 were run close to EOR temperatures (395-405°C) to accelerate deactivation. The hydro- gen to oil ratio, hydrogen purity and total operating pressure remained the same for all test conditions. In addition, as was the case with heat- ing blocks 1 and 2, catalysts were finally tested by a repeat condition using LVGO feed to evaluate deactivation (see Table 2). In this repeat condition, the catalyst from each vendor can now be assessed at both normal as well as accelerated deactivation conditions.
Results and discussion
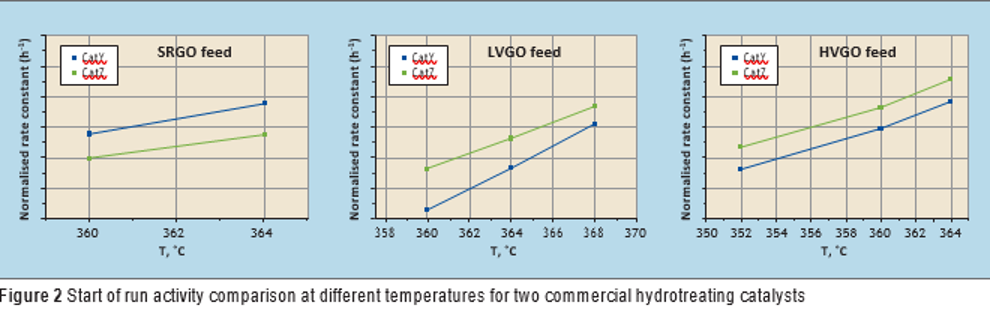
Catalyst testing (blocks 1 and 2) was carried out around the target effluent sulphur concentration for different types of feed (see Table 2) by adjusting the temperature. A pseudo first order kinetic plug flow reactor model was used to model the hydrodesulphurisation reaction rate obtained at different temperatures and using different feeds. The apparent first order rate constant for the catalysts at different temperatures was calculated as follows and compared for each feed (see Figure 2):
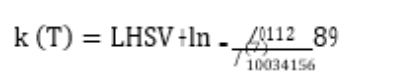
where k(T) is the apparent first order reaction rate constant, Cfeed is the sulphur concentration in the feed, LHSV is the liquid hourly space velocity, and CTeffluent is the concentration of sulphar in the effluent at T temperature.
For LVGO and HVGO feeds, CatZ showed better activity compared to CatY (see Figure 2). However, in the case of SRGO feed, CatY showed higher activity compared to CatZ at similar process conditions (see Figure 2). It seems that CatY is better designed for middle distillates with lower density feed but lower functionality towards removal of the refractory types of sulphur species present in heavier hydrocarbons, or other properties related to catalyst design, both of which were outside the scope of this study.
It is interesting to see how performance evaluation can change based on the type of feedstock with the same catalysts. This underlines the value of catalyst testing and using actual feed and process conditions for an accurate comparison of catalysts. By analysis of concentration of aromatics in the liquid effluent, no significant difference in hydrogen consumption was observed between the two catalysts (CatY and CatZ).
To evaluate catalyst deactivation, LVGO feed was used during the first and the last stage of catalyst testing under the same conditions.
At SOR conditions (blocks 1 and 2), CatY and CatZ experienced different feeds and temperature in the range 352-368°C (see Table 2). The sulphur concentration in the effluent at the end of catalyst testing (TOS =30 days) compared to the sulphur concentration at the start (TOS = 4 days) for each catalyst were at similar levels (see Figure 3a). This indicates that, after 30 days of operation at SOR
temperatures, it is difficult to measure catalyst deactivation for diesel hydrodesulphurisation catalyst. This was expected with typical deactivation rates of around 0.5-2°C of WABT per month. In parallel reactors containing CatY and CatZ catalysts (blocks3 and 4), catalysts were tested at a higher temperature (EOR). With different feeds and actual EOR conditions (395-405°C), both catalysts deactivated significantly during the test period. Sulphur concentration in the effluents of CatY and CatZ at the start (time on stream [TOS] = 4 days) was significantly lower than during the last stage (TOS = 30 days). CatZ is better in terms of stability compared to CatY, as effluent sulphur in the case of CatZ is much lower than for CatY at TOS = 30 days
(see Figure 3b).
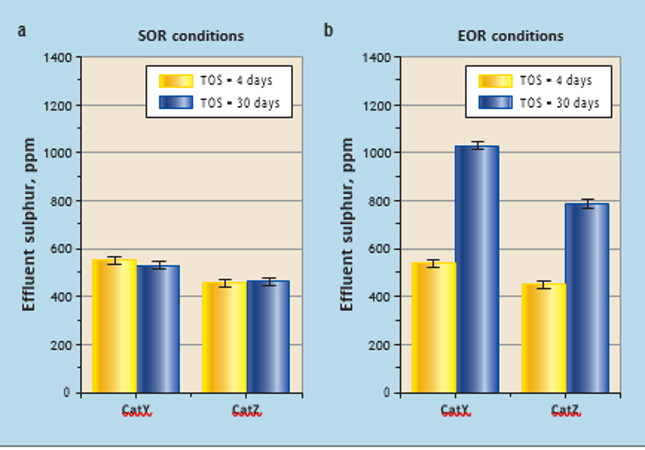
Figure 3 Catalyst deactivation by comparing sulphur concentration during repeat conditions at start and end of run using LVGO feed: (a) start of run conditions experienced by the catalysts; (b) End of run conditions experienced by the catalysts reactor). This section of the bed can be used to compare the deactivation experienced at lab scale.
Testing at EOR temperatures, which are within the actual temperature range of commercial operation, is a good choice to evaluate deactivation rates in a short period of time. As the commercial reactor operates in an adiabatic mode, the temperature increases along the length of the bed. This means that, even at the SOR conditions of a commercial reactor, the lower catalyst bed in the reactor will also experience higher temperatures (depending on the temperature rise in the reactor). This section of the bed can be used to compare the deactivation experienced at lab scale.
As discussed above, the most likely cause of deactivation in processing middle distillates and feeds with low metal content is coke formation. Over its lifetime, a catalyst may experience some sintering of active sites. This is one of the reasons why the activity of a regenerated catalyst is significantly restored after coke removal.6 Around 80-90% of the hydrodesulphurisation and hydrodenitrification activity is typically restored with a simple coke burn-off, depending on how the commercial middle distillate (without Ni/V in feed) hydrotreating reactor was operated during its lifetime.2 High temperature conditions thermodynamically favour formation of polyaromatic species and less adsorption of hydrogen on the catalyst surface, which leads to coke formation on the catalyst. In a 30-day period with EOR temperature conditions, significant (12-15°C) deactivation was observed in both catalysts (CatY and CatZ). However, CatZ clearly performs better in terms of stability.
Conclusion
A 30-day time-on-stream operation allows a clear comparison of activity and stability of commercial hydrotreating catalysts by applying an effective experimental design in a parallel reactor unit. CatZ is better in terms of stability and activity for LVGO and HVGO desulphurisation. A change of feed type and composition can change the ranking of catalyst in terms of activity. The catalyst ages around 4-5 times faster than in normal operation when operated at EOR conditions. This allows ranking of catalysts on their stability, which is not possible in a SOR activity test run for a period of one month.
To further evaluate deactivation, it is important to compare the coke profile in the accelerated (within normal operating temperature range) aged catalyst with spent catalyst from a commercial reactor taken at the appropriate position. This can further validate accelerated ageing of hydrotreating catalysts.
References
1 Hydroprocessing catalyst deactivation in
commercial practice, Catalysis Today 154, 2010, 256-263.
2 Handbook of Spent Hydroprocessing Catalysts 2010, Ch 6, 121-122, Elsevier ISBN 978-0-444-53556-6.
3 Accelerated deactivation studies of hydrotreating catalysts in pilot plant, Applied Catalysis A, General 548, 2017, 114-121.
4 Accelerated deactivation of hydrotreating catalysts: comparison to long-term deactivation in a commercial plant, Catalysis Today 45, 1998, 319-325.
5 Life cycle stability of ULSD catalysts, PTQ Catalysis, 2019, 1-5.
6 Decrease catalyst costs by regeneration, analysis and sorting, PTQ Catalysis, 2012, Article no 1000352.
Abrar Hakeem is a Scientist with Q8 Research, Europoort, Rotterdam, The Netherlands. He is responsible for hydroprocessing catalyst refinery applications and holds a master of technology degree in chemical engineering from IIT Kanpur and a PhD in catalysis engineering from Delft University of Technology, The Netherlands. Email: abhakeem@q8.com
Ed Ouwerkerk is a Hydrotreating Consultant with Catalyst Intelligence SARL. He has worked for over 30 years in oil refining and holds a MSc in experimental physics from the University of Leiden and a PhD in molecular physics from the University of Amsterdam, The Netherlands. Email: ouwerkerk@catalyst-intelligence.com
| |

